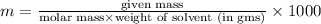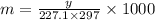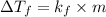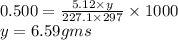Chemistry, 08.07.2019 22:00 adrianty8496
The freezing point of benzene c6h6 is 5.50°c at 1 atmosphere. a nonvolatile, nonelectrolyte that dissolves in benzene is tnt (trinitrotoluene). how many grams of tnt, c7h5n3o6 (227.1 g/mol), must be dissolved in 297.0 grams of benzene to reduce the freezing point by 0.500°c ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
The freezing point of benzene c6h6 is 5.50°c at 1 atmosphere. a nonvolatile, nonelectrolyte that dis...
Questions


Mathematics, 24.01.2020 23:31


Mathematics, 24.01.2020 23:31

Physics, 24.01.2020 23:31



History, 24.01.2020 23:31

Mathematics, 24.01.2020 23:31

Physics, 24.01.2020 23:31


Mathematics, 24.01.2020 23:31





Health, 24.01.2020 23:31

Mathematics, 24.01.2020 23:31

English, 24.01.2020 23:31

Mathematics, 24.01.2020 23:31

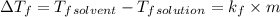
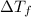 = 0.500°C
= 0.500°C 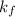 = for benzene is 5.12
= for benzene is 5.12