
Chemistry, 08.07.2019 23:00 stefancvorovic1
Asmall diamond, which is made of pure carbon, contains too many carbon atoms to count individually. which is closest to the number of carbon atoms in a 1.0-g diamond? disabled a. 3.6 x 10–45 carbon atoms student selected incorrect b. 9.2 x 101 carbon atoms disabled c. 5.0 x 1022 carbon atoms disabled d. 4,509 carbon atoms

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Asmall diamond, which is made of pure carbon, contains too many carbon atoms to count individually....
Questions

Health, 21.06.2019 23:00

Mathematics, 21.06.2019 23:00

Mathematics, 21.06.2019 23:00





History, 21.06.2019 23:00

Mathematics, 21.06.2019 23:00




English, 21.06.2019 23:00

Mathematics, 21.06.2019 23:00


Mathematics, 21.06.2019 23:00

Mathematics, 21.06.2019 23:00


Mathematics, 21.06.2019 23:00

Mathematics, 21.06.2019 23:00

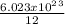 C atoms = 5.02 x 10²² C atoms
C atoms = 5.02 x 10²² C atoms

