
Chemistry, 09.07.2019 03:00 itscheesycheedar
Look at the image below: which of the following statements is true? a. the figure is an atom and an element. b. the figure is a molecule and a compound. c. the figure is a molecule and an element. d. the figure is a compound, but not a molecule.
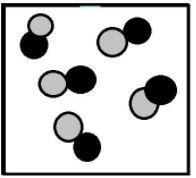

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Look at the image below: which of the following statements is true? a. the figure is an atom and a...
Questions

Business, 08.07.2019 23:00



Mathematics, 08.07.2019 23:00

History, 08.07.2019 23:00

Biology, 08.07.2019 23:00

Mathematics, 08.07.2019 23:00

Biology, 08.07.2019 23:00


Biology, 08.07.2019 23:00

Mathematics, 08.07.2019 23:00

Chemistry, 08.07.2019 23:00

Business, 08.07.2019 23:00



English, 08.07.2019 23:10

History, 08.07.2019 23:10


History, 08.07.2019 23:10



