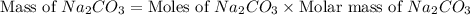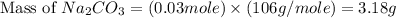Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Sodium hydroxide reacts with carbon dioxide to form sodium carbonate and water: 2 naoh(s) + co2(g)...
Questions

Mathematics, 02.05.2021 17:00


English, 02.05.2021 17:00

Mathematics, 02.05.2021 17:00

History, 02.05.2021 17:00

Mathematics, 02.05.2021 17:00


Physics, 02.05.2021 17:00


Mathematics, 02.05.2021 17:10



Mathematics, 02.05.2021 17:10

Mathematics, 02.05.2021 17:10


Spanish, 02.05.2021 17:10


World Languages, 02.05.2021 17:10


Mathematics, 02.05.2021 17:10

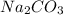 prepared can be 3.18 grams.
prepared can be 3.18 grams.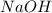 .
.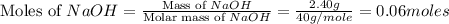
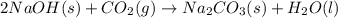
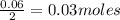 of
of