
Chemistry, 09.07.2019 04:30 ryrytkg5107
Consider the reaction cl2(g) + i2(g) < => 2 icl(g), which is endothermic as written. what would be the effect on the equilibrium position of removing cl2(g)? 1. reaction would go to the left, making more "reactants" 2. no change on the equilibrium position 3. reaction would go to the right, making more "reactants" 4. reaction would go to the left, making more "products" 5. reaction would go to the right, making more "products"

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

You know the right answer?
Consider the reaction cl2(g) + i2(g) < => 2 icl(g), which is endothermic as written. what wou...
Questions


History, 07.04.2021 15:30


Mathematics, 07.04.2021 15:30

French, 07.04.2021 15:30




Engineering, 07.04.2021 15:30


Mathematics, 07.04.2021 15:30

History, 07.04.2021 15:30



Spanish, 07.04.2021 15:30




Mathematics, 07.04.2021 15:30

Biology, 07.04.2021 15:30

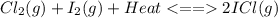
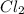 is removed from the system, the concentration of chlorine (
is removed from the system, the concentration of chlorine (

