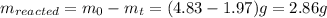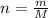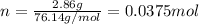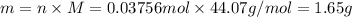Chemistry, 09.07.2019 04:30 romeojose2005
The decomposition reaction of carbon disulfide to carbon monosulfide and sulfur is first order with k = 2.80 ✕ ✕ 10−7 sec-1 at 1000°c. cs2(g) → cs(g) + s(g) a. how much of a 4.83-gram sample of carbon disulfide would remain after 37.0 days? 1.97 1.97 grams carbon disulfide b. how much carbon monosulfide would be formed after 37.0 days? 1.14 1.65 grams carbon monosulfide useful information 1.013 bar = 760 torr = 1 atm = 760 mm hg

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 00:30
Lem 2 the data below are for the system ethyl propyl ether (1)-chloroform (2) at 0.5 bar. use the data to answer the following questions (all questions refer to p d 0: 5 bar). a) what are the boiling points of the pure components at 0.5 bar? b) a mixture with the overall composition z1 d 0: 1 is brought to 47.6ä±c, 0.5 bar. what is the phase? c) 100 mole of a mixture with z1 d 0: 1 (state a) is mixed with 22 mole of pure ethyl propyl ether vapor (state b). the mixing takes place at 47.6 ä±c, 0.5. bar. what is the phase of the resulting mixture (state c)? if the state is a v/l mixture report the number of moles and mole fractions in each phase. d) plot the txy graph and show states a, b and c. the graph must be done by computer and should be properly annotated. ethyl propyl ether (1) - chloroform (2) at 0.5 bar t ( ä±c) x1 y1 t ( ä±c) x1 y1 42.9 0.000 0.000 49.0 0.470 0.455 43.0 0.020 0.010 49.1 0.520 0.520 43.9 0.065 0.029 48.9 0.567 0.592 45.4 0.156 0.089 48.3 0.652 0.720 46.4 0.215 0.142 47.6 0.745 0.815 47.6 0.296 0.223 46.7 0.822 0.872 48.3 0.362 0.302 45.7 0.907 0.937 48.7 0.410 0.375 44.6 1.000
Answers: 3

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
The decomposition reaction of carbon disulfide to carbon monosulfide and sulfur is first order with...
Questions

Mathematics, 14.07.2019 00:30





Mathematics, 14.07.2019 00:30

Mathematics, 14.07.2019 00:30




Mathematics, 14.07.2019 00:30

Mathematics, 14.07.2019 00:30




Mathematics, 14.07.2019 00:30


Mathematics, 14.07.2019 00:30

Mathematics, 14.07.2019 00:30

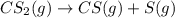
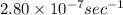 .
.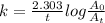
![[A_{0}]](/tpl/images/0068/1392/747e3.png) is initial concentration of reactant and
is initial concentration of reactant and ![[A_{t}]](/tpl/images/0068/1392/b9281.png) is concentration at time t.
is concentration at time t.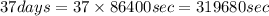
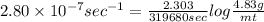
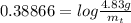
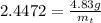
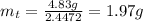
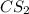 gives 1 mol of CS.
gives 1 mol of CS.