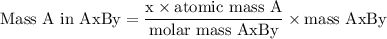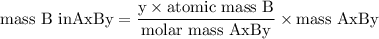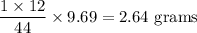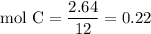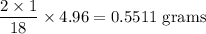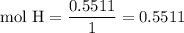Chemistry, 09.07.2019 07:30 kitttimothy55
Complete combustion of 3.20g of a hydrocarbon produced 9.69g of co2 and 4.96g of h2o. what is the empirical formula for the hydrocarbon?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
Complete combustion of 3.20g of a hydrocarbon produced 9.69g of co2 and 4.96g of h2o. what is the em...
Questions

English, 23.11.2021 17:40

Arts, 23.11.2021 17:40


Physics, 23.11.2021 17:40

Mathematics, 23.11.2021 17:40

English, 23.11.2021 17:40




History, 23.11.2021 17:40



Biology, 23.11.2021 17:40



English, 23.11.2021 17:50

Mathematics, 23.11.2021 17:50

Biology, 23.11.2021 17:50

Mathematics, 23.11.2021 17:50

Mathematics, 23.11.2021 17:50