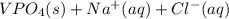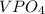Chemistry, 09.07.2019 08:00 aaronlikly
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rules to identify the composition of the salt that precipitates out of the solution. a. vpo4 b. na3po4 c. vcl3 d. nacl

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rule...
Questions


Physics, 30.03.2021 20:00

Social Studies, 30.03.2021 20:00

Mathematics, 30.03.2021 20:00


Mathematics, 30.03.2021 20:00

Mathematics, 30.03.2021 20:00


Mathematics, 30.03.2021 20:00






Chemistry, 30.03.2021 20:00


Mathematics, 30.03.2021 20:00

History, 30.03.2021 20:00

Chemistry, 30.03.2021 20:10

Social Studies, 30.03.2021 20:10

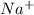 ,
,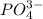 ,
,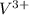 , and
, and 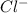 . The products formed in the reaction would be NaCl and VPO
. The products formed in the reaction would be NaCl and VPO . As per the solubiltiy rules, all chloride salts are soluble, all phosphate salts are insoluble except those with
. As per the solubiltiy rules, all chloride salts are soluble, all phosphate salts are insoluble except those with 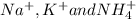 .
.