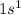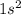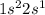Chemistry, 09.07.2019 12:00 loravillanueva87
Which set of these comparisons is incorrect? (more stable means it has a more negative energy.) a. the 1s orbital in h is more stable than the 1s orbital in he+ b. the 2s orbital in he atom is less stable than the 2s orbital in he+ c. the 2s subshell in li is more stable than the 2p subshell in li?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3


Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Which set of these comparisons is incorrect? (more stable means it has a more negative energy.) a....
Questions








Mathematics, 26.10.2019 02:43

Mathematics, 26.10.2019 02:43

Social Studies, 26.10.2019 02:43

Chemistry, 26.10.2019 02:43



Mathematics, 26.10.2019 02:43

History, 26.10.2019 02:43


Mathematics, 26.10.2019 02:43



Mathematics, 26.10.2019 02:43