
Chemistry, 20.12.2019 17:31 caraxphernelia
Tia has a sample of pure gold (au). she weighed the sample and the result was 35.9 grams. tia wants to determine the number of atoms in the sample. calculate the number of atoms in 35.9 g of pure gold.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 07:30
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
Tia has a sample of pure gold (au). she weighed the sample and the result was 35.9 grams. tia wants...
Questions







Social Studies, 25.11.2021 14:00





Social Studies, 25.11.2021 14:00


English, 25.11.2021 14:00

Geography, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00

Social Studies, 25.11.2021 14:00

Business, 25.11.2021 14:00


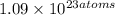 of gold.
of gold.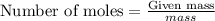
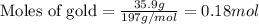
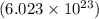 of particles.
of particles.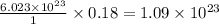 atoms of gold.
atoms of gold.

