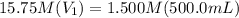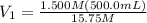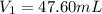Chemistry, 09.07.2019 13:30 genyjoannerubiera
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist with 10.00 l of 15.75 m perchloric acid solution to prepare the required solution. calculate the volume of concentrated acid required.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist wi...
Questions

Health, 27.05.2021 14:00

Mathematics, 27.05.2021 14:00


Mathematics, 27.05.2021 14:00

French, 27.05.2021 14:00


English, 27.05.2021 14:00

Physics, 27.05.2021 14:00

English, 27.05.2021 14:00




Business, 27.05.2021 14:00



English, 27.05.2021 14:00



Business, 27.05.2021 14:00

French, 27.05.2021 14:00

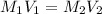
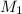 is the concentration of the concentrated solution and
is the concentration of the concentrated solution and 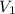 is it's volume.
is it's volume. 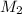 is the concentration of the diluted solution and
is the concentration of the diluted solution and 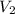 is it's volume. Let's plug in the values in the equation and solve it for
is it's volume. Let's plug in the values in the equation and solve it for