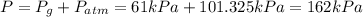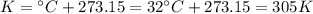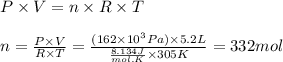Chemistry, 09.07.2019 15:30 josie17340
Larisa pumps up a soccer ball until it has a gauge pressure of 61 kilopascals. the volume of the ball is 5.2 liters. the air temperature is 32°c, and the outside air is at standard pressure. how many moles of air are in the ball?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
Larisa pumps up a soccer ball until it has a gauge pressure of 61 kilopascals. the volume of the bal...
Questions

Mathematics, 21.05.2021 19:00

Mathematics, 21.05.2021 19:00

Spanish, 21.05.2021 19:00

Biology, 21.05.2021 19:00






Mathematics, 21.05.2021 19:00


Mathematics, 21.05.2021 19:00

History, 21.05.2021 19:00


Mathematics, 21.05.2021 19:00


Mathematics, 21.05.2021 19:00

Mathematics, 21.05.2021 19:00

Mathematics, 21.05.2021 19:00