
Be sure to answer all parts. industrially, hydrogen gas can be prepared by combining propane gas (c3h8) with steam at about 400°c. the products are carbon monoxide (co) and hydrogen gas (h2). (a) write a balanced equation for the reaction. include phase abbreviations. (b) how many kilograms of h2 can be obtained from 8.31 × 103 kg of propane

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Be sure to answer all parts. industrially, hydrogen gas can be prepared by combining propane gas (c3...
Questions


English, 08.12.2020 07:10

Biology, 08.12.2020 07:10


SAT, 08.12.2020 07:10


Mathematics, 08.12.2020 07:10

Mathematics, 08.12.2020 07:10



Social Studies, 08.12.2020 07:10



Mathematics, 08.12.2020 07:10

Chemistry, 08.12.2020 07:10



History, 08.12.2020 07:10

Mathematics, 08.12.2020 07:10

Mathematics, 08.12.2020 07:10

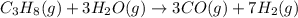
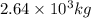
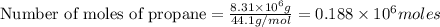
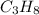 gives 7 moles of
gives 7 moles of 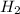
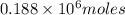 moles of
moles of 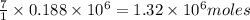 of
of 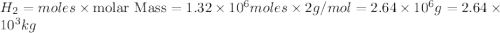
 kg of propane
kg of propane

