Chemistry, 09.07.2019 17:00 oliviastarkweather16
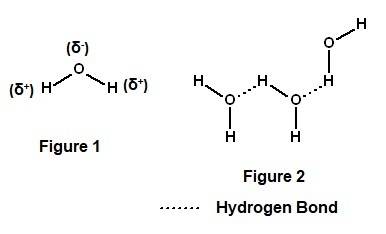
Water molecules have a polarity, which allows them to be electrically attracted to other water molecules and other polar molecules by weak chemical bonds known as view available hint(s) water molecules have a polarity, which allows them to be electrically attracted to other water molecules and other polar molecules by weak chemical bonds known as hydrogen bonds polar covalent bonds van der waals interactions ionic bonds nonpolar covalent bonds

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Water molecules have a polarity, which allows them to be electrically attracted to other water molec...
Questions

Mathematics, 26.11.2019 03:31

Mathematics, 26.11.2019 03:31


Spanish, 26.11.2019 03:31


Mathematics, 26.11.2019 03:31

Social Studies, 26.11.2019 03:31


English, 26.11.2019 03:31

History, 26.11.2019 03:31

Chemistry, 26.11.2019 03:31


Mathematics, 26.11.2019 03:31



Mathematics, 26.11.2019 03:31



Chemistry, 26.11.2019 03:31