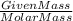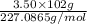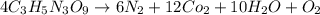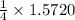Chemistry, 09.07.2019 17:00 mathwiznot45
Be sure to answer all parts. nitroglycerin (c3h5n3o9) is a powerful explosive. its decomposition may be represented by 4c3h5n3o9 → 6n2 12co2 10h2o o2 this reaction generates a large amount of heat and gaseous products. it is the sudden formation of these gases, together with their rapid expansion, that produces the explosion. (a) what is the maximum amount of o2 in grams that can be obtained from 3.50 × 102 g of nitroglycerin

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 12:30
Atriple covalent bond involves two atoms sharing three pairs of electrons. true false
Answers: 2
You know the right answer?
Be sure to answer all parts. nitroglycerin (c3h5n3o9) is a powerful explosive. its decomposition may...
Questions





English, 14.04.2020 21:53

Mathematics, 14.04.2020 21:53







Mathematics, 14.04.2020 21:53


English, 14.04.2020 21:53





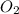 obtained = 12.576 g
obtained = 12.576 g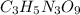 = 3.50 × 102g
= 3.50 × 102g