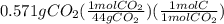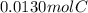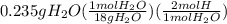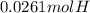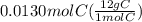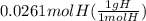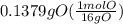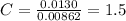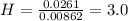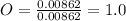Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
A0.320 g sample of a carboxylic acid is burned in oxygen, producing 0.571 g of co2 and 0.235 g of h2...
Questions


History, 13.08.2019 04:20

Mathematics, 13.08.2019 04:20



Mathematics, 13.08.2019 04:20

Physics, 13.08.2019 04:20

Mathematics, 13.08.2019 04:20

Chemistry, 13.08.2019 04:20


Mathematics, 13.08.2019 04:20

English, 13.08.2019 04:20


Mathematics, 13.08.2019 04:20


Mathematics, 13.08.2019 04:20




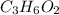 .
.