
Amixture of babr2 and inert material is analyzed to determine the ba content. first the mixture is dissolved in water. then all of the bromide ion is precipitated as agbr by the addition of an excess of silver nitrate. babr2(> 2agbr(s)+ba(no3)2(aq). in one experiment a 0.7207 g sample of the mixture resulted in 0.6226 g of agbr. determine the percent (by mass) of ba in the mixture

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
Amixture of babr2 and inert material is analyzed to determine the ba content. first the mixture is d...
Questions



English, 24.08.2019 04:10

Mathematics, 24.08.2019 04:10


Mathematics, 24.08.2019 04:10

Mathematics, 24.08.2019 04:10

Physics, 24.08.2019 04:10




Mathematics, 24.08.2019 04:10

Mathematics, 24.08.2019 04:10

Mathematics, 24.08.2019 04:10

English, 24.08.2019 04:10





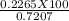 =31.42 %
.
=31.42 %
.

