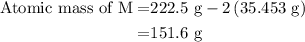Chemistry, 09.07.2019 21:00 balwinderdev
A0.999-g sample of a metal chloride, mcl2, is dissolved in water and treated with excess aqueous silver nitrate. the silver chloride that formed weighed 1.286 g. calculate the atomic mass of m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
A0.999-g sample of a metal chloride, mcl2, is dissolved in water and treated with excess aqueous sil...
Questions


English, 01.12.2020 19:40

Mathematics, 01.12.2020 19:40

Health, 01.12.2020 19:40

Mathematics, 01.12.2020 19:40

Social Studies, 01.12.2020 19:40


Health, 01.12.2020 19:40


Chemistry, 01.12.2020 19:40


Biology, 01.12.2020 19:40

Business, 01.12.2020 19:40

English, 01.12.2020 19:40

Mathematics, 01.12.2020 19:40


Chemistry, 01.12.2020 19:40

Mathematics, 01.12.2020 19:40

Mathematics, 01.12.2020 19:40

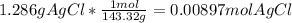

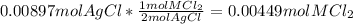
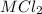 =0.999 g
=0.999 g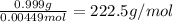
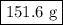 .
.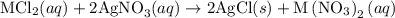
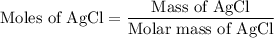 ...... (1)
...... (1)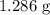 for mass of AgCl and
for mass of AgCl and 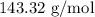 for molar mass of AgCl in equation (1).
for molar mass of AgCl in equation (1).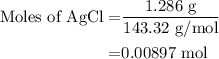
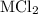 reacts with two moles of
reacts with two moles of 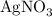 to form two moles of AgCl and one mole of
to form two moles of AgCl and one mole of 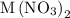 , So stoichiometric ratio between
, So stoichiometric ratio between 
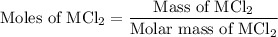 ...... (2)
...... (2)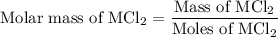 ...... (3)
...... (3)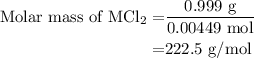
![\text{Molar mass of MCl}_2=\left[1\left(\text{Atomic mass of M}\right)+\\\\2\left(\text{Atomic mass of Cl}\right)\right]}](/tpl/images/0070/7913/9c07b.png) ....... (4)
....... (4)![\text{Atomic mass of M}=\left[\text{Molar mass of MCl}_2-2\left(\text{Atomic mass of Cl}\right)\right]](/tpl/images/0070/7913/5c489.png) ...... (5)
...... (5)