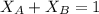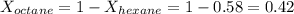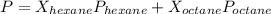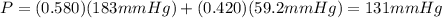Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2

You know the right answer?
At a given temperature the vapor pressures of hexane and octane are 183 mmhg and 59.2 mmhg, respecti...
Questions


Mathematics, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00


Biology, 30.10.2020 01:00




Chemistry, 30.10.2020 01:00




History, 30.10.2020 01:00


Mathematics, 30.10.2020 01:00


Social Studies, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00

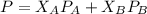
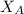 is mole fraction of A,
is mole fraction of A, 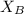 is mole fraction of B,
is mole fraction of B, 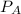 is partial pressure of A and
is partial pressure of A and 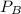 is partial pressure of B.
is partial pressure of B.