
Chemistry, 10.07.2019 00:30 chrisraptorofficial
Which statement about reversible reactions is correct? at equilibrium, the forward and reverse reactions stop at the appropriate concentrations. at equilibrium, the forward and reverse reactions continue indefinitely. at equilibrium the rate of reaction of products divided by the rate of reaction of reactants equal the equilibrium constant, k.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Which statement about reversible reactions is correct? at equilibrium, the forward and reverse reac...
Questions

Geography, 04.07.2019 15:30

Physics, 04.07.2019 15:30




Social Studies, 04.07.2019 15:30


Computers and Technology, 04.07.2019 15:30



Advanced Placement (AP), 04.07.2019 15:30


Spanish, 04.07.2019 15:30



Spanish, 04.07.2019 15:30




Mathematics, 04.07.2019 15:30

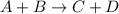
![k = \frac{[C][D]}{[A][B]}](/tpl/images/0071/3436/05b05.png)
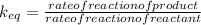
![\frac{\frac{d[C][D]}{dt}}{\frac{d[A][B]}{dt}}](/tpl/images/0071/3436/6ae5f.png)


