

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Calculate the mean free path of air molecules at 3.50 * 10-13 atm and 300 k. (this pressure is readi...
Questions


Mathematics, 06.02.2022 20:40


German, 06.02.2022 20:40



Geography, 06.02.2022 20:40



Chemistry, 06.02.2022 20:40

Mathematics, 06.02.2022 20:40

Mathematics, 06.02.2022 20:40

English, 06.02.2022 20:40


Biology, 06.02.2022 20:40


Business, 06.02.2022 20:40


History, 06.02.2022 20:50

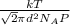 ---------(1)
---------(1)

