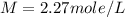Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Procaine hydrochloride ( = 272.77 g/mol) is used as a local anesthetic. calculate the molarity of a...
Questions

Mathematics, 08.07.2019 08:00

Mathematics, 08.07.2019 08:00


Mathematics, 08.07.2019 08:00

Mathematics, 08.07.2019 08:00



Mathematics, 08.07.2019 08:00



Mathematics, 08.07.2019 08:00

Mathematics, 08.07.2019 08:00




Mathematics, 08.07.2019 08:00

History, 08.07.2019 08:00

Mathematics, 08.07.2019 08:00

Mathematics, 08.07.2019 08:00

Mathematics, 08.07.2019 08:00

![d=M[\frac{1}{m}+\frac{M_b}{1000}]](/tpl/images/0071/7962/a25dd.png)
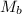 = molar mass of solute (Procaine hydrochloride) = 272.77 g/mole
= molar mass of solute (Procaine hydrochloride) = 272.77 g/mole![1.1066=M[\frac{1}{4.66}+\frac{272.77}{1000}]](/tpl/images/0071/7962/29bbf.png)