
Chemistry, 10.07.2019 03:30 jadeandryna0609
The vapor pressure of benzene, c6h6, is 100.0 torr at 26.1 °c. assuming raoult's law is obeyed, how many moles of a nonvolatile solute must be added to 100.0 ml of benzene to decrease its vapor pressure by 10.0% at 26.1 °c? the density of benzene is 0.8765 g> cm3.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Monkeys and bats have similar bone structure in their forelimbs. however, monkeys have longer forelimbs to use for climbing and swinging in trees. bats have shorter forelimbs to use for flight. which term best describes how monkey and bat forelimbs are related to each other? a. homologous b. embryonic c. analogous d. vestigial
Answers: 1


Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
The vapor pressure of benzene, c6h6, is 100.0 torr at 26.1 °c. assuming raoult's law is obeyed, how...
Questions

English, 02.08.2019 15:00

Mathematics, 02.08.2019 15:00





Mathematics, 02.08.2019 15:00

Health, 02.08.2019 15:00

Mathematics, 02.08.2019 15:00






History, 02.08.2019 15:00




Physics, 02.08.2019 15:00

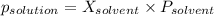 -(1)
-(1)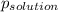 is observed vapor pressure of the solution,
is observed vapor pressure of the solution, 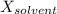 is mole fraction of solvent, and
is mole fraction of solvent, and 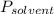 is vapor pressure of the pure solvent.
is vapor pressure of the pure solvent.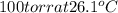 (given)
(given)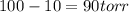
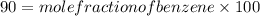
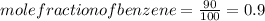
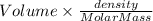
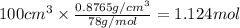
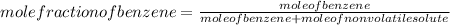
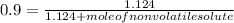
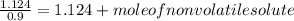
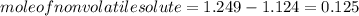
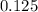 .
.

