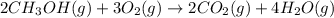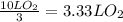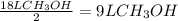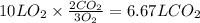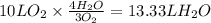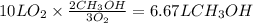Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Estructura 7.2 completar repaso 1 - ¿lógico o ilógico? 1 - ¿lógico o ilógico? listen and indicate whether each question and response is lógico or ilógico.
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2


Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
Consider the combustion of methanol at some high temperature in a constant-pressure reaction chamber...
Questions

Mathematics, 10.07.2019 15:00

Mathematics, 10.07.2019 15:00

Biology, 10.07.2019 15:00

Mathematics, 10.07.2019 15:00


Mathematics, 10.07.2019 15:00


Social Studies, 10.07.2019 15:00

Mathematics, 10.07.2019 15:00


Mathematics, 10.07.2019 15:00





Mathematics, 10.07.2019 15:00


History, 10.07.2019 15:00

Mathematics, 10.07.2019 15:00

Biology, 10.07.2019 15:00