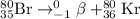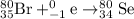Chemistry, 10.07.2019 05:30 22savage2017
He 80br (atomic number 35) nuclide decays either by β− decay or by electron capture. (masses of atoms: 80br =79.918528 amu; 80kr =79.916380 amu; 80se =79.916520 amu. neglect the mass of electrons involved because these are atomic, not nuclear, masses.) (a) write the balanced nuclear equations for each process below. use the isotope tool in the palette to enter both the mass numbers and the atomic numbers for each nuclide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
He 80br (atomic number 35) nuclide decays either by β− decay or by electron capture. (masses of atom...
Questions

Biology, 11.03.2020 09:20



Biology, 11.03.2020 09:21

Chemistry, 11.03.2020 09:22



English, 11.03.2020 09:26

Biology, 11.03.2020 09:26


Chemistry, 11.03.2020 09:27

Mathematics, 11.03.2020 09:27



English, 11.03.2020 09:28



Chemistry, 11.03.2020 09:28


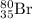 by
by 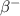 decay is as follows:
decay is as follows: