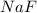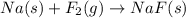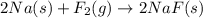The fluoride rinse in dental offices usually contains sodium fluoride. sodium fluoride can be prepared from the reaction between sodium metal and fluorine gas. which properly represents the balanced chemical equation for this reaction? na(s) + f2(g) → naf2(s) na(s) + f(g) → naf(s) 7na(s) + f(g) → na7f(s) 2na(s) + f2(g) → 2na2f(s) 2na(s) + f2(g) → 2naf(s) why is it important to understand this equation? what do you think could happen if this is incorrect? g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
The fluoride rinse in dental offices usually contains sodium fluoride. sodium fluoride can be prepar...
Questions



English, 10.06.2021 19:40


Mathematics, 10.06.2021 19:40



Mathematics, 10.06.2021 19:40


English, 10.06.2021 19:40






Mathematics, 10.06.2021 19:40




 .
.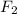 .
.