Chemistry, 10.07.2019 05:30 Hrjohnson2004
Identify the limiting reactant in the reaction of hydrogen and ethylene (c2h4) to form c2h6, of 4.40 g of h2 and 10.5 g of c2h4 are combined. determine the amount (in grams) of excess reactant that remains after the reaction is complete.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Identify the limiting reactant in the reaction of hydrogen and ethylene (c2h4) to form c2h6, of 4.40...
Questions


Computers and Technology, 19.03.2021 03:00



Mathematics, 19.03.2021 03:00


Biology, 19.03.2021 03:00







Mathematics, 19.03.2021 03:00


English, 19.03.2021 03:00


History, 19.03.2021 03:00


Social Studies, 19.03.2021 03:00

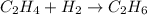
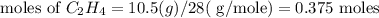
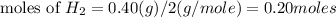
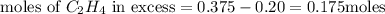
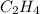 is the limiting reagent.
is the limiting reagent.