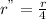Chemistry, 10.07.2019 05:30 jessie8022
Is first order in bro3⎻ , second order in br⎻, and zero order in h+. by what factor will the reaction rate change if the concentration of bro3⎻ is doubled, the concentration of br⎻ is halved, and the concentration of h+ is tripled ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
Is first order in bro3⎻ , second order in br⎻, and zero order in h+. by what factor will the reactio...
Questions

History, 28.06.2019 20:20

Mathematics, 28.06.2019 20:20

Mathematics, 28.06.2019 20:20


History, 28.06.2019 20:20


Spanish, 28.06.2019 20:20




Mathematics, 28.06.2019 20:20

Mathematics, 28.06.2019 20:20





English, 28.06.2019 20:20



![r=k[A]^{a}[B]^{b}](/tpl/images/0072/1558/66bfa.png)
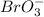 , second order with respect to
, second order with respect to 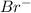 and zero order with respect to
and zero order with respect to 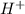 .
.![r=k[BrO_{3}^{-}]^{1}[Br^{-}]^{2}[H^{+}]^{0}](/tpl/images/0072/1558/0a846.png)
![r=k[BrO_{3}^{-}][Br^{-}]^{2}](/tpl/images/0072/1558/30a30.png) ...... (1)
...... (1)![r^{'}=2k[BrO_{3}^{-}][Br^{-}]^{2}](/tpl/images/0072/1558/4b879.png) ...... (2)
...... (2)![\frac{r^{'}}{r}=\frac{2k[BrO_{3}^{-}][Br^{-}]^{2}}{k[BrO_{3}^{-}][Br^{-}]^{2}}=2](/tpl/images/0072/1558/5cd5e.png)
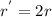
^{2}](/tpl/images/0072/1558/6cb53.png)
![r^{"}=1/4k[BrO_{3}^{-}][Br^{-}]^{2}](/tpl/images/0072/1558/92754.png) ...... (3)
...... (3)![\frac{r^{"}}{r}=\frac{1/4k[BrO_{3}^{-}][Br^{-}]^{2}}{k[BrO_{3}^{-}][Br^{-}]^{2}}=\frac{1}{4}](/tpl/images/0072/1558/807de.png)