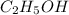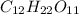Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrol...
Questions


Biology, 26.01.2021 22:50





Mathematics, 26.01.2021 22:50

English, 26.01.2021 22:50




Mathematics, 26.01.2021 22:50



Physics, 26.01.2021 22:50

Mathematics, 26.01.2021 22:50


Mathematics, 26.01.2021 22:50


English, 26.01.2021 22:50

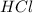
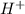 and
and 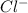 ) and thus, it is a strong electrolyte.
) and thus, it is a strong electrolyte.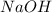
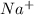 and
and 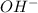 ) and thus, it is a strong electrolyte.
) and thus, it is a strong electrolyte.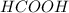
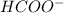 ) and thus, it is a weak electrolyte.
) and thus, it is a weak electrolyte.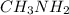
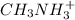 and
and 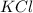
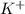 and
and