Chemistry, 10.07.2019 06:00 Meap12345678910
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 3.75 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 3.75 ∘c. what is the molal concentration...
Questions


Social Studies, 08.12.2021 21:50






English, 08.12.2021 21:50






Mathematics, 08.12.2021 21:50



Mathematics, 08.12.2021 21:50


Social Studies, 08.12.2021 21:50

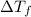 can be calculated as follows:
can be calculated as follows: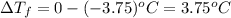
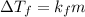
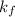 is freezing point depression constant.
is freezing point depression constant.