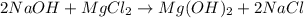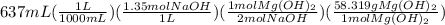Sodium hydroxide and magnesium chloride react as shown by this equation: 2naoh + mgcl2 → mg(oh)2 + 2nacl. suppose the reaction begins with 637 milliliters of 1.35 m sodium hydroxide solution and excess magnesium hydroxide. what is the theoretical yield of magnesium hydroxide if the resulting solution has a volume of 2.82 liters? use the periodic table and the polyatomic ion resource. the mass of magnesium hydroxide formed is grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
Sodium hydroxide and magnesium chloride react as shown by this equation: 2naoh + mgcl2 → mg(oh)2 +...
Questions
















Mathematics, 20.08.2019 01:10