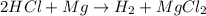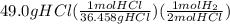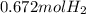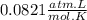When magnesium reacts with hydrochloric acid, hydrogen gas is formed: 2hcl + mg → h2 + mgcl2. what is the volume of hydrogen produced at 25°c and 101.3 kilopascals when 49.0 grams of hcl reacts with excess magnesium? use the periodic table and ideal gas resource. a. 1.38 l b. 2.76 l c. 16.4 l d. 32.9 l e. 33.1 l

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

You know the right answer?
When magnesium reacts with hydrochloric acid, hydrogen gas is formed: 2hcl + mg → h2 + mgcl2. what...
Questions

SAT, 30.11.2021 03:50




Mathematics, 30.11.2021 03:50




Chemistry, 30.11.2021 03:50


Geography, 30.11.2021 04:00

Computers and Technology, 30.11.2021 04:00