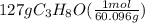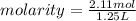Chemistry, 10.07.2019 07:30 sduhaime1974
Isopropanol (c3h8o) is a key ingredient in some hand sanitizers. suppose that 127 grams of isopropanol is dissolved in water. the volume of the solution is 1,250 milliliters. what is the molarity of the solution? refer to the periodic table to you answer. express your answer to three significant figures. the molarity of the solution is m.

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:02
If the empirical formula of a compound is known what is needed in order to determine the molecular formula a) the coordination numbers b)the molecular geometry c) the molar mass
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
Isopropanol (c3h8o) is a key ingredient in some hand sanitizers. suppose that 127 grams of isopropan...
Questions

Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00





Chemistry, 12.12.2020 17:00


Physics, 12.12.2020 17:00

English, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

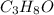 = 3(12.011) + 8(1.008) + 1(15.999) = 60.096 gram per mol
= 3(12.011) + 8(1.008) + 1(15.999) = 60.096 gram per mol