Chemistry, 10.07.2019 08:30 tybreyonnaHco7855
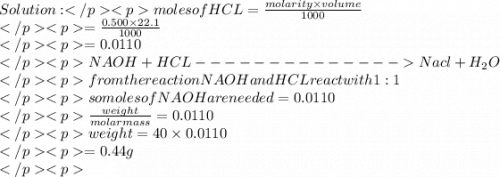
Asample contains both naoh and nacl. 0.500 g of this sample was dissolved in water to make a 20.0 ml solution and then this solution was titrated by 0.500 mol/l hcl solution. if 22.1 ml of hcl was used to reach the end point, what is the mass % of naoh in the sample?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
Asample contains both naoh and nacl. 0.500 g of this sample was dissolved in water to make a 20.0 ml...
Questions

Mathematics, 22.01.2021 05:40

History, 22.01.2021 05:40

History, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40





Mathematics, 22.01.2021 05:40


Mathematics, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40

History, 22.01.2021 05:40


Business, 22.01.2021 05:40


Arts, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40

English, 22.01.2021 05:40