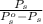An ideal solution is formed from a mixture of the nonvolatile solute urea, co(nh2)2, and methanol, ch3oh. the vapor pressure of pure methanol at 20°c is 89 mmhg. if 6.0 g of urea is mixed with 39.8 g of methanol, calculate the vapor pressure of the methanol solution.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

You know the right answer?
An ideal solution is formed from a mixture of the nonvolatile solute urea, co(nh2)2, and methanol, c...
Questions

English, 15.04.2021 15:50






Mathematics, 15.04.2021 15:50

Mathematics, 15.04.2021 15:50


Mathematics, 15.04.2021 15:50


Mathematics, 15.04.2021 15:50



Mathematics, 15.04.2021 15:50


Mathematics, 15.04.2021 15:50