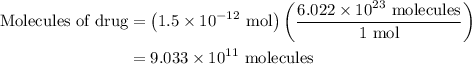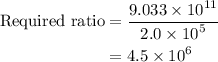Amedical lab is testing a new anticancer drug on cancer cells. the drug stock solution concentration is 1.5×10−9m, and 1.00 ml of this solution will be delivered to a dish containing 2.0×105 cancer cells in 5.00 ml of aqueous fluid. what is the ratio of drug molecules to the number of cancer cells in the dish?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Amedical lab is testing a new anticancer drug on cancer cells. the drug stock solution concentration...
Questions




Mathematics, 20.01.2021 18:00


Mathematics, 20.01.2021 18:00

Mathematics, 20.01.2021 18:00

English, 20.01.2021 18:00

Mathematics, 20.01.2021 18:00

Mathematics, 20.01.2021 18:00

History, 20.01.2021 18:00

World Languages, 20.01.2021 18:00




Mathematics, 20.01.2021 18:00

Spanish, 20.01.2021 18:00

Mathematics, 20.01.2021 18:00


Chemistry, 20.01.2021 18:00

 .
.
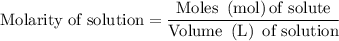 …… (1)
…… (1)
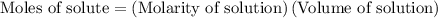 …… (2)
…… (2)
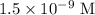 for molarity of solution and 1.00 mL for volume of solution in equation (2) to calculate moles of drug.
for molarity of solution and 1.00 mL for volume of solution in equation (2) to calculate moles of drug.
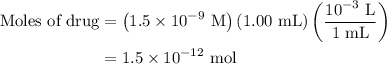
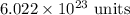 . These units can vary from question to question and can be atoms, molecules, or formula units. Since one mole of drug also contains
. These units can vary from question to question and can be atoms, molecules, or formula units. Since one mole of drug also contains 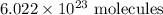 , number of molecules of drug present in
, number of molecules of drug present in 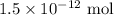 can be calculated as follows:
can be calculated as follows: