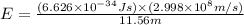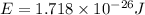Chemistry, 01.10.2019 20:30 kyramks421
Calculate the energy of a photon of wavelength 11.56 meters. (planck’s constant is 6.626 x 10-34 joule seconds; the speed of light is 2.998 x 108 m/s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Calculate the energy of a photon of wavelength 11.56 meters. (planck’s constant is 6.626 x 10-34 jou...
Questions

History, 24.10.2020 01:50



Mathematics, 24.10.2020 01:50

Mathematics, 24.10.2020 01:50









Business, 24.10.2020 01:50


Computers and Technology, 24.10.2020 01:50

Mathematics, 24.10.2020 01:50


Mathematics, 24.10.2020 01:50

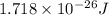
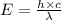
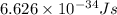
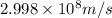
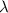 = wavelength = 11.56 m
= wavelength = 11.56 m