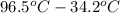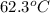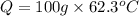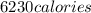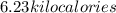Chemistry, 10.07.2019 20:30 johajackson2014
How many kilocalories are required to heat 100.00 g of water from 34.2°c to 96.5°c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3


Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
How many kilocalories are required to heat 100.00 g of water from 34.2°c to 96.5°c?...
Questions

English, 11.03.2021 02:50

Computers and Technology, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50



Mathematics, 11.03.2021 02:50



Mathematics, 11.03.2021 02:50





Mathematics, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50


Mathematics, 11.03.2021 02:50


Mathematics, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50

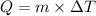
 = change in temperature in degree Celsius
= change in temperature in degree Celsius