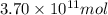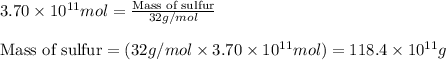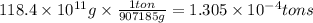Chemistry, 10.07.2019 21:00 hapjajsjjz3738
The annual production of sulfur dioxide from burning coal and fossil fuels, auto exhaust, and other sources is about 26 million tons. the equation for the reaction is s(s) + o2(g) → so2(g) how much sulfur (in tons), present in the original materials, would result in that quantity of so2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
The annual production of sulfur dioxide from burning coal and fossil fuels, auto exhaust, and other...
Questions



Mathematics, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00


Mathematics, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00


English, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00



Mathematics, 24.04.2021 01:10



Mathematics, 24.04.2021 01:10

Chemistry, 24.04.2021 01:10

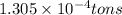
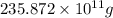 (Conversion factor:
(Conversion factor: 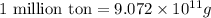 )
)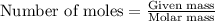 .....(1)
.....(1)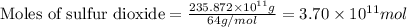
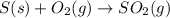
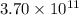 moles of sulfur dioxide will be produced by =
moles of sulfur dioxide will be produced by = 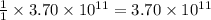 moles of sulfur.
moles of sulfur.