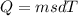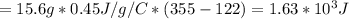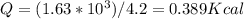Chemistry, 11.07.2019 04:00 yselahernandez02
How many kilocalories are required to increase the temperature of 15.6 g of iron from 122 °c to 355 °c. the specific heat of iron is 0.450 j/g °c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
How many kilocalories are required to increase the temperature of 15.6 g of iron from 122 °c to 355...
Questions




Mathematics, 14.10.2020 22:01


History, 14.10.2020 22:01

Mathematics, 14.10.2020 22:01


Mathematics, 14.10.2020 22:01


Advanced Placement (AP), 14.10.2020 22:01

Mathematics, 14.10.2020 22:01


Mathematics, 14.10.2020 22:01





Mathematics, 14.10.2020 22:01