
This reaction shows the complete combustion of octane, c8h18, a component of gasoline. 2 c8h18(l) + 25 o2(g) 16 co2(g) + 18 h2o(l) (a) how many moles of o2 are needed to burn 1.60 mol of c8h18? mol (b) how many moles of co2 are produced when 0.19 mol of c8h18 are burned? mol (c) how many grams of o2 are needed to burn 1.15 g of c8h18? g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
This reaction shows the complete combustion of octane, c8h18, a component of gasoline. 2 c8h18(l) +...
Questions



Geography, 08.04.2021 22:20

Biology, 08.04.2021 22:20






Mathematics, 08.04.2021 22:20





Mathematics, 08.04.2021 22:20

Mathematics, 08.04.2021 22:20



English, 08.04.2021 22:20

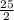 O₂→ 8 CO₂ + 9 H₂O.
O₂→ 8 CO₂ + 9 H₂O. 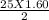 mole= 20 moles.
mole= 20 moles.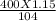 g= 4.42 g.
g= 4.42 g.

