
Chemistry, 11.07.2019 07:30 djdkfkfkckmfmf7724
Amixture contains only nacl and al2(so4)3. a 1.45 g sample of the mixture is dissolved in water, and an excess of naoh is added, producing a precipitate of al(oh)3. the precipitate is filtered, dried, and weighed. the mass of the precipitate is 0.495 g. what is the mass percent of al2(so4)3 in the sample?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3


Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1

Chemistry, 23.06.2019 14:00
[08.05] which answer provides the correct name for name the following hydrocarbon? (2 points) moving left to right: a hydrocarbon chain made of a methyl group (ch subscript three) single bond carbon triple bond carbon single bond ch bonded to a methyl group (ch subscript three) and a chain including a methelyne group (ch subscript two) single bond methyl group (ch subscript three). 4-ethyl-2-pentyne 4-methyl-2-hexyne 2-heptyne 4-methy-hexane
Answers: 1
You know the right answer?
Amixture contains only nacl and al2(so4)3. a 1.45 g sample of the mixture is dissolved in water, and...
Questions

Mathematics, 20.05.2020 23:06

Mathematics, 20.05.2020 23:06

Health, 20.05.2020 23:06

Mathematics, 20.05.2020 23:06



English, 20.05.2020 23:06

World Languages, 20.05.2020 23:06

Mathematics, 20.05.2020 23:06

Mathematics, 20.05.2020 23:06







Social Studies, 20.05.2020 23:06

Mathematics, 20.05.2020 23:06

Geography, 20.05.2020 23:06

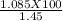 =74.8 %.
=74.8 %.

