
Chemistry, 11.07.2019 07:30 j1theking18
Acompound contains only carbon, hydrogen, and oxygen. combustion of 11.75 mg of the compound yields 17.61 mg co2 and 4.81 mg h2o. the molar mass of the compound is 176.1 g/mol. what are the empirical and molecular formulas of the compound? (type your answer using the format co2 for co2.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1


You know the right answer?
Acompound contains only carbon, hydrogen, and oxygen. combustion of 11.75 mg of the compound yields...
Questions


Mathematics, 16.12.2020 18:30

Mathematics, 16.12.2020 18:30


Mathematics, 16.12.2020 18:30

Biology, 16.12.2020 18:30

Social Studies, 16.12.2020 18:30




Mathematics, 16.12.2020 18:30

Mathematics, 16.12.2020 18:30


Mathematics, 16.12.2020 18:30



Chemistry, 16.12.2020 18:30

Computers and Technology, 16.12.2020 18:30

Mathematics, 16.12.2020 18:30

Physics, 16.12.2020 18:30

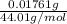
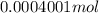
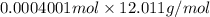
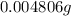
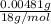

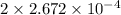
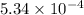
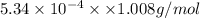
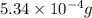
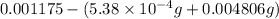
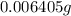
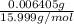
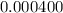
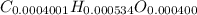
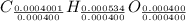
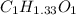
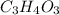 (empirical fomula)
(empirical fomula)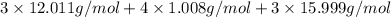
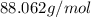
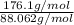
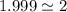
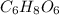
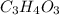 and molecular formula is
and molecular formula is 

