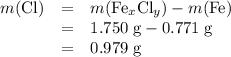Chemistry, 11.07.2019 11:00 hflores0001
Chloe t. followed the procedure of this experiment to determine the empirical formula of a compound of iron (fe) and chlorine (cl). to do so, she added 2.15 g of zn to a solution containing 1.750 g of fe(x)cl(y). after the reaction was complete, she isolated 0.771 g of fe. the mass of cl in the fe(x)cl(y) solution is

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
Chloe t. followed the procedure of this experiment to determine the empirical formula of a compound...
Questions

French, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40


Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

History, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

English, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

English, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

English, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Social Studies, 26.01.2021 17:40

 sample contains only the atoms of
sample contains only the atoms of 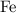 , andChlorine,
, andChlorine, 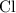
 and
and 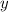 are,
are, 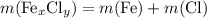 shall holds.
shall holds.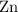 is more reactive than iron as seen in the reactivity series; Adding zinc to the solution would reduce all iron atoms (regardless of the oxidation state,
is more reactive than iron as seen in the reactivity series; Adding zinc to the solution would reduce all iron atoms (regardless of the oxidation state, 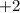 or
or 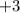 ) to their elementary form, hence displacing them out of the solution.
) to their elementary form, hence displacing them out of the solution. 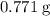 of iron was obtained and therefore
of iron was obtained and therefore 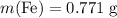 .
.