
Chemistry, 11.07.2019 11:30 LindaCat78
Zinc metal reacts with hydrochloric acid according to the balanced equation: zn(s) + 2 hcl(aq) ¡ zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.5 °c to 23.7 °c. find ∆hrxn for this reaction as written. (use 1.0 g/ml for the

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1


Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
Zinc metal reacts with hydrochloric acid according to the balanced equation: zn(s) + 2 hcl(aq) ¡ zn...
Questions

Social Studies, 29.03.2021 17:30


Biology, 29.03.2021 17:30

Mathematics, 29.03.2021 17:30


Computers and Technology, 29.03.2021 17:30



Mathematics, 29.03.2021 17:30


Mathematics, 29.03.2021 17:30


English, 29.03.2021 17:30



Spanish, 29.03.2021 17:30


Mathematics, 29.03.2021 17:30


Biology, 29.03.2021 17:30

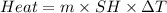
 is change in temperature.
is change in temperature.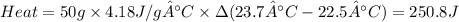
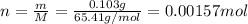
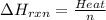
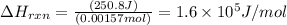
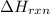 for the given reaction is
for the given reaction is 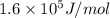
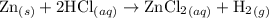
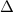 T,
T,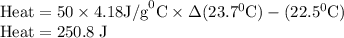
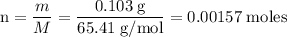
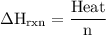
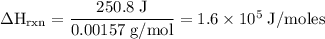
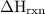 =
= 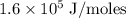 for the given equation.
for the given equation. 

