
If the same quantity of heat is added to both a 2-liter and a 4-liter container of water, the temperature change for water in the 4-liter container will be a. half that of the 2-liter container. b. more than half but less than twice. c. twice that of the 2-liter container. d. no change. explain.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of sulfur dioxide are in 2.26 × 10^33 sulfur dioxide molecules?
Answers: 3

You know the right answer?
If the same quantity of heat is added to both a 2-liter and a 4-liter container of water, the temper...
Questions



History, 11.10.2019 04:30



Mathematics, 11.10.2019 04:30

English, 11.10.2019 04:30

SAT, 11.10.2019 04:30

Mathematics, 11.10.2019 04:30


Mathematics, 11.10.2019 04:30



Chemistry, 11.10.2019 04:30


English, 11.10.2019 04:30

Biology, 11.10.2019 04:30



Mathematics, 11.10.2019 04:30

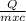
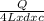
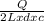
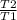 = 2
= 2


