
Chemistry, 11.07.2019 15:00 wittlemarie
At a certain temperature, the solubility of n2 gas in water at 3.08 atm is 72.5 mg of n2 gas/100 g water . calculate the solubility of n2 gas in water, at the same temperature, if the partial pressure of n2 gas over the solution is increased from 3.08 atm to 8.00 atm . express your answer numerically to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
At a certain temperature, the solubility of n2 gas in water at 3.08 atm is 72.5 mg of n2 gas/100 g w...
Questions

History, 27.06.2019 01:00



Chemistry, 27.06.2019 01:00





Mathematics, 27.06.2019 01:00

Mathematics, 27.06.2019 01:00


Mathematics, 27.06.2019 01:00

Mathematics, 27.06.2019 01:00








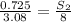
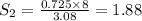
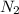 gas in 1 g of water or, 188 mg of tex]N_{2}[/tex] gas in 100 g of water.
gas in 1 g of water or, 188 mg of tex]N_{2}[/tex] gas in 100 g of water.


