
Bromine reacts with nitric oxide to form nitrosyl bromide as shown in this reaction: br2(g) + 2 no(g) → 2 nobr(g) a possible mechanism for this overall reaction is shown below. no(g) + br2(g) br2(g) (fast step; keq = k1/k−1) k2 nobr(g) + no(g) → 2 nobr(g) (slow step) what is the rate law for formation of nobr in terms of reactants based on this mechanism

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

You know the right answer?
Bromine reacts with nitric oxide to form nitrosyl bromide as shown in this reaction: br2(g) + 2 no(...
Questions


Mathematics, 11.03.2021 14:00

Biology, 11.03.2021 14:00



Biology, 11.03.2021 14:00


Mathematics, 11.03.2021 14:00

Social Studies, 11.03.2021 14:00





Mathematics, 11.03.2021 14:00

Arts, 11.03.2021 14:00

Biology, 11.03.2021 14:00

Biology, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00

English, 11.03.2021 14:00

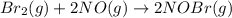
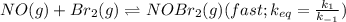
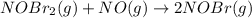 (slow step
(slow step 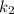 )
)![r_{1}=k_{1}[NO][Br_{2}]-k_{-1}[NOBr_{2}]](/tpl/images/0077/5023/9bc26.png)
![r_{2}=k_{2}[NOBr_{2}] [NO]](/tpl/images/0077/5023/6e659.png)
![[NOBr_{2}]](/tpl/images/0077/5023/48931.png) takes place in this reaction.
takes place in this reaction.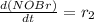
![k_{2}[NOBr_{2}][NO]](/tpl/images/0077/5023/433e8.png) (1)
(1)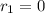
![k_{1}[NO][Br_{2}]= k_{-1}[NOBr_{2}]](/tpl/images/0077/5023/8345a.png)
![[NOBr_{2}] = \frac{k_{1}}{k_{-1}}[NO][Br_{2}]](/tpl/images/0077/5023/ee42f.png)
![\frac{d(NOBr)}{dt}=k_{2}[NOBr_{2}][NO]](/tpl/images/0077/5023/ff99b.png)
![k_{2} \frac{k_{1}}{k_{-1}}[NO][Br_{2}][NO]](/tpl/images/0077/5023/f5111.png)
![\frac{k_{1}k_{2}}{k_{-1}}[NO]^{2}[Br_{2}]](/tpl/images/0077/5023/427a8.png)
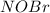 in terms of reactants is given by
in terms of reactants is given by 

