
Choose the correct interpretation of this result in terms of the law of definite proportion. the composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. the composition of a substance depends on the numbers of atoms of each element making up the compound (depends on the formula of the compound) and not on the composition of the mixture from which it was formed. the composition of a substance depends on the composition of the mixture from which it was formed and not on the numbers of atoms of each element making up the compound (not on the formula of the compound). the composition of a mixture depends on the numbers of atoms of each element making up the mixture and not on the composition of the substance from which it was formed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2


Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
Choose the correct interpretation of this result in terms of the law of definite proportion. the com...
Questions






Mathematics, 12.03.2021 15:10






Computers and Technology, 12.03.2021 15:10

Computers and Technology, 12.03.2021 15:10







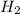 (g) +
(g) + 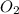 (g) = 2
(g) = 2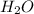 (l). Here the origin of the components i.e. hydrogen and oxygen does not matter it depends upon the number of atoms present in the reactant and product. Thus among all the other interpretations- The composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. This is correct.
(l). Here the origin of the components i.e. hydrogen and oxygen does not matter it depends upon the number of atoms present in the reactant and product. Thus among all the other interpretations- The composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. This is correct. 

