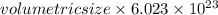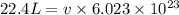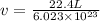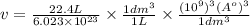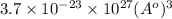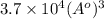Calculate the volumetric size of a water molecule in water vapor at normal conditions, assuming 1 mole of the vapor occupies 22.4 l, as if the vapor were an ideal gas. give answer in angstroms, two significant digits. do not write down units in your answer. calculate the volumetric size of a water molecule in water vapor at normal conditions, assuming 1 mole of the vapor occupies 22.4 l, as if the vapor were an ideal gas. give answer in angstroms, two significant digits. do not write down units in your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Calculate the volumetric size of a water molecule in water vapor at normal conditions, assuming 1 mo...
Questions


Mathematics, 12.03.2021 20:20

History, 12.03.2021 20:20

Mathematics, 12.03.2021 20:20



Mathematics, 12.03.2021 20:20





English, 12.03.2021 20:20




History, 12.03.2021 20:20


History, 12.03.2021 20:20

Mathematics, 12.03.2021 20:20

Mathematics, 12.03.2021 20:20

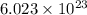 molecules of water.
molecules of water.